I’ve posted about basic biochemistry before, focusing on how macronutrients (fats, carbohydrates, and proteins) are used by the body. However, the existence of macro nutrients implies the existence of micronutrients, which are additional substances that you have to eat to survive, but in much smaller quantities. In this post I’ll explore fatty acids, cholesterol, antioxidants, and electrolytes–what they are, and how they work.
Fatty Acids
You’ve probably heard of omega-3 fatty acids, the main component of the giant translucent yellow pills that make your breath smell fishy. You may also have heard of omega-6 fatty acids, in the context of keeping their ratio compared to the omega-3s low. But what are they? What do they do?
A fatty acid is a molecule consisting of an acid on one end and a lipid chain (a bunch of carbon atoms in a line with hydrogen atoms sticking out to the sides) on the other. It’s one type of biologically relevant lipid, with the others being sterols (including cholesterol–more on that later), phospholipids (which famously make up a majority of cell membranes and play an important role in the RNA World Hypothesis), and waxes (such as beeswax, spermaceti, and lanolin (wool oil)). (Earwax technically isn’t a wax, but is made mostly of shed skin and fatty acids.) There are other types of lipids as well, as usually happens when humans try to categorize things, but those are the main groups. Fatty acids are the main component of all fats that we eat and are made of. So, many types of fatty acid are macronutrients, and are metabolized the way I explained in my previous post.
However, essential fatty acids are fatty acids that can’t be synthesized by the body, but are still required for biological functioning. As with essential amino acids and vitamins as I discussed in my previous post, which ones are essential varies from one animal to the next, but for humans only two types of fatty acid are essential: linoleic acid (an omega-6 fatty acid), and alpha-linoleic acid (an omega-3 fatty acid). The term omega-x is just a shorthand way of describing the fatty acid’s molecular structure. Omega refers to the end of the chain, and the number counts how many carbon atoms away the first carbon-carbon double bond occurs. So, there are many types of omega-3 and omega-6 fatty acids, but only one of each is an essential fatty acid. However, the omega-3 that’s found in fish oil pills is a different one, called timnodonic acid, which isn’t technically an essential fatty acid as it can be converted in the body from alpha-linoleic acid, but in practice that happens in such tiny amounts that you’re better off just eating it anyway.
Fun fat fact 1: The name “linoleic” comes from the fact that they were first isolated from linseed (or flaxseed) oil. Linoleum, the uibiquitous crappy flooring material, was originally made from canvas covered in solidified linseed oil, but is now more often vinyl plastic.
Fun fat fact 2: The two essential fatty acids, when discovered, were originally called “vitamin F”, but for some reason this name was abandoned.
So what do omega-3 and omega-6 fatty acids do in the body? Their two most important functions are regulating inflammation and the endocannabinoid system. The former is well-understood, while the second is barely understood at all, but is certainly very important.
Inflammation is a generalized injury-fighting response in which blood vessels in the affected area swell and become more permeable, bringing more blood to the area and allowing it to leak out into the surrounding non-vessel tissues. Eventually, the tissues become clogged with blood, causing redness and swelling, but the stopped bloodflow acts as a temporary “train station” that allows white blood cells to enter and exit the bloodstream, and go fight pathogens in the affected tissues. To effectively fight infection and heal a wound (or to do any biological function), there are lots of steps that need to be coordinated to happen at the same time. So, it makes sense that many related tasks should share a common cause, collectively called a “cascade”. Omega-6 fatty acids cause the cascade that promotes inflammation, while omega-3s cause the one that inhibits it. That’s why the ratio of one to the other, rather than the absolute amount of either, is important–they directly cancel each other’s effects. Both are necessary in order to survive, but there’s a time and place for inflammation, and if your ratio of omega-6s to omega-3s gets too lopsided in either direction, it can cause a problem. The more common problem in the modern day is too much omega-6 relative to omega-3, which correlates with chronic inflammation, which disrupts normal biological functioning. (Higher levels of pollutants and irritants in the modern day also correlate with chronic inflammation. So it’s not clear which cause, environment or diet, is more to blame, or if they’re both necessary for this condition.)
The endocannabinoid system consists of particular neurotransmitters and the receptors throughout the central and peripheral nervous system that they bind to. As you’ve probably guessed, these receptors accept THC, the active chemical in marijuana, and it’s because of marijuana that the whole system of endocannabinoids was discovered. Scientists could see THC fitting neatly into a receptor, but why would such a receptor exist if the only thing that fit into it came from outside the body? There must be something that those receptors had evolved for. Eventually, they isolated the endocannabinoid known as anandamide in 1999, and it was followed by the discovery of a bunch more. Some of these are made from linoleic acid. It’s unclear exactly how the endocannabinoid system works, but it’s known to play a role in such diverse functions as fertility, appetite, pain, mood, memory, and more.
Cholesterol
Whew, that was a long section. Let’s cover the other important type of fat now before moving on to other types of molecules. Cholesterol is also made of a bunch of hydrogen and carbon atoms, plus one oxygen atom, but rather than being a chain it has a structure that includes four fused rings. This four-ring structure is what defines a steroid, a class of lipids that you’ve probably heard of.
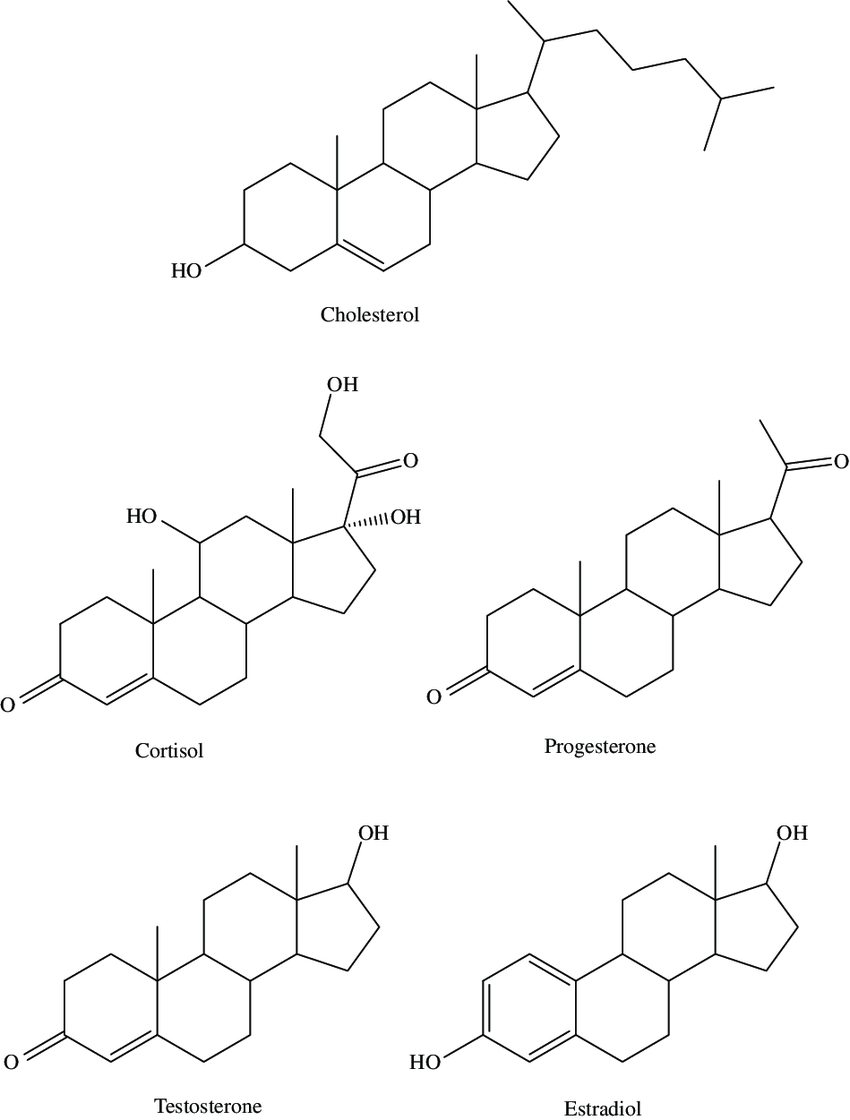
Cholesterol is an important component of cell membranes, in which its role is to keep the membrane supple across a range of conditions. All animal cells synthesize it out of simple metabolic materials in a complicated, many-step process, so even if you don’t eat any cholesterol, your body will make enough of it to function. If you eat lots of cholesterol, your body notices and reduces the amount it makes over the next few hours. It’s a myth that eating too many eggs causes high cholesterol levels. Here’s a comic that explains the following two paragraphs in a more fun way, if you prefer.
In humans, most of our cholesterol production happens in the liver and intestines, but every cell needs it to maintain its membrane, so it must be efficiently transported throughout the body. The molecules that perform this task are called lipoproteins (cholesterol can’t circulate unchaperoned since it’s nonpolar, like most fats, and thus doesn’t mix with the water that makes up the majority of blood). Lipoproteins are categorized by density, ranging from ultra-low-density lipoprotein to high-density lipoprotein. The denser the protein, the more balled-up and small of a package it carries its cholesterol in, which we’ll come back to. The two most important lipoproteins are low-density lipoprotein, which carries cholesterol from the liver to cells that need it, and high-density lipoprotein, which carries cholesterol from cells with extra back to the liver. These proteins circulate around the bloodstream until a needy cell flags one of them down by putting a receptor for that protein into its membrane.
However, sometimes low-density lipoprotein, as it’s fluffier than high-density lipoprotein, gets stuck when moving through small vessels. When lots of them get stuck, they block the blood flow, and can cause heart attacks and other issues. High-density lipoprotein can reverse the buildup. That’s why you often hear low-density lipoprotein, or LDL, nicknamed “Bad Cholesterol” and high-density lipoprotein, or HDL, nicknamed “Good Cholesterol”. This is very misleading, as neither of them are actually cholesterol.
In addition to its use in cell membranes, cholesterol is transformed into every steroid hormone humans use, including testosterone, estrogen, progesterone, and cortisol, as well as vitamin D and bile acid. I’m not sure if the versatile nature of cholesterol is why we use steroids as chemical signals–it’s useful for other functions, ubiquitous in the body, and easy to modify–or if there are other reasons they make good hormones.
Antioxidants
Unlike cholesterol and fatty acids, antioxidants don’t belong to one class of molecule, but rather are united by their function. They react with dangerous particles called “reactive oxygen species”, or ROS, such as hydrogen peroxide (hair bleach), hydroxyl, and superoxide, which are by-products of normal metabolism, and prevent them from damaging important structures. These ROS are why highly concentrated oxygen is toxic–it’s a very reactive element, which unchecked would go around reacting with molecules that are not supposed to change, like DNA and cell membranes. The body can safely dispose of some amount of ROS, but it can be overwhelmed. Other conditions than too much oxygen can also cause this condition, which is known as “oxidative stress”, such as sunburn, smoking, or starving. However, ROS aren’t only negative–they have uses as chemical signals and as weapons against pathogens, so there’s an opposite malady known as “antioxidative stress”, in which an overload of antioxidants can make the immune system less effective and interrupt certain cellular signaling. Oxidative stress is much more common though, and by damaging DNA, ROS can cause cells to become cancerous, which is why antioxidants are touted as preventing cancer.
While there’s a lot of marketing nowadays calling things “packed with antioxidants”, there are only three known substances that act as antioxidants when you eat them: vitamins A, C, and E. So it’s actually really easy to determine how much antioxidant is in something–just look at the nutrition label. It’s sometimes been claimed that polyphenols are also dietary antioxidants, but more recent evidence shows that they only have antioxidant properties in certain situations, of which the human body isn’t one.
Electrolytes
Now we’re into the realm of minerals–heavier chemical elements, rather than compounds of common elements, that need to be eaten in trace amounts, as they can’t be synthesized in the body (that would require alchemy). Essential minerals for humans include calcium (whose crystals are interwoven with protein strands to make bones), iron (used in the proteins hemoglobin and myoglobin, which store oxygen in blood and muscles, respectively), and electrolytes.
An electrolyte is any substance that when dissolved in a polar solvent like water, conducts electricity through chemical reactions. Wait, let’s back up and define all those terms. What’s a polar solvent? A polar molecule is one that has a different electric charge on one end than the other, due to unequal distribution of electrons. Water is polar, as its two hydrogen atoms each give their electron to the oxygen atom, leaving the hydrogen atoms positively charged and the oxygen negatively charged. Water is a polar solvent because the forces it exerts on other materials due to being polar can pull those substances apart, or dissolve them. For example, table salt consists of one sodium atom and one chlorine atom. The sodium atom donates its spare electron to the chlorine atom, becoming positively charged while the chlorine atom becomes negatively charged; then, since opposites attract, they stick together. However, when you put salt in water, the charged ends of the water molecules pull harder than the sodium and chlorine atom pull on each other, forming little “red rover” spheres of water molecules surrounding a salt molecule, with the water molecules all facing in or facing away depending on the charge of the atom they’re surrounding.
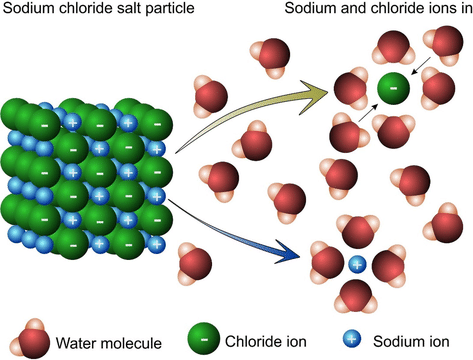
This is why eating salty food makes you thirsty–the dissolution of the salt in your body’s water means that the water molecules surrounding the salt atoms are not accessible for other uses.
Now that the charged particles are separated, they can move around independently, allowing the overall solution to be electrically charged. If you put the salt water in an electric field, all the sodium would move to one side and the chlorine would move to the other. However, holding a charge and conducting electricity are two separate things–the latter requires the movement of electrons. Salt water can do that too, but it’s a bit more complicated.
In salt water, adding electrons at one end breaks apart water molecules into hydroxyl (OH), which can accept electrons, and hydrogen gas, and taking electrons out at the other end removes them from the chloride ions, which makes the chlorine atoms stick together into chlorine gas. Then, the sodium ions, which are still positively charged, react with the hydroxyl ions, which are negatively charged, to form bleach. Thus, through the flow of electrons through simple salt water, three dangerous substances are created. And electrons will stop flowing once any of the inputs runs out. Electricity is sometimes just chemistry!
Anyway, other elements on the extreme ends of the periodic table, such as calcium, magnesium, and potassium, are also important electrolytes for biology. Some enzymes require the presence of an electrolyte to catalyze the reaction they’re supposed to perform, and muscles and nerves work by bringing electrolytes in or out of the cell, changing their charge. That’s a whole other blog post. (Or textbook.) This is why people who run ultra-marathons have to eat salt tablets in addition to drinking lots of water–if you don’t have enough electrolytes, your muscles and nerves don’t work well.
Why do we sweat out electrolytes then? Seems inefficient. Well, the body does its best to resorb as many electrolytes as possible from sweat before it is excreted, and the sodium content of sweat is less than a third of the sodium content of blood plasma, where the liquid comes from. But moving things against diffusion is hard, so we end up losing some amount in sweat. That’s the price of effective cooling, and evolution has decided it’s worth it, at least for humans and horses.
Conclusion
Were you surprised by any of these tidbits? How about the fact that only a tenth of your skeleton’s weight is calcium? Weird, right?
Anyway, I hope you learned something about bio terms you’ve heard of but not explored deeply. Now if someone asks you, “What is an antioxidant, anyway?” you’ll have an answer. And you can warn them not to go overboard with those antioxidants or they might put themselves in antioxidative stress.


